business
Foods with Health Claims development support business(Food CRO)
In Foods with Health Claims development support business(Food CRO), we have racked up achievements as an expert in food since 2001.
We emphasis the speed and compliance of food development, propose the clinical trials plan, prepare the protocol, manage clinical trials , and analyze data to support your smooth research and development.
In addition, we support development of Foods for Specified Health Use (TOKUHO) and Foods with Functional Claims.
Don’t worry about the function and safety evaluation of food.
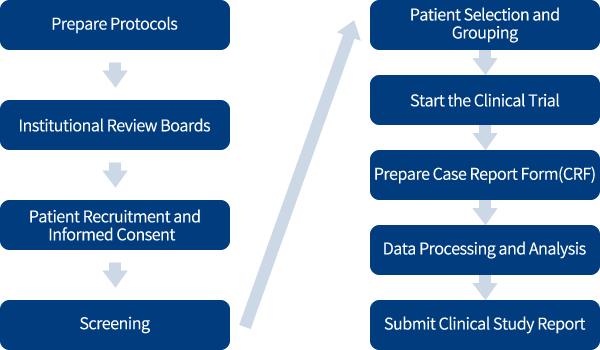
Study Commissioned Business
In Foods with Health Claims development support business(Food CRO), we have been entrusted with over 1480 human clinical trials and about 800 non-clinical study of food product. Moreover, over 280 journal have been published since 2001.
We have had about 500 companies including international company, food company, mail order and e-commerce company, raw material suppliers, cosmetic company, and more.
Based on our wide experience, we propose the best studies to your needs, such as applying for TOKUHO, notification of Foods with Functional Claims, preparing articles, and more.
Human Clinical Trial
We utilize networks of patients and clinical sites located all over Japan.
As we welcome famous statistician and Medical Experts as advisers, we provide higher quality and precise service.
We evaluate the effectiveness of food, cosmetics, exercise equipment, beauty equipment, relaxation tool, and more., for human.
Basic Research and Animal Testing
We perform in vivo and/or in vitro evaluation of functionality and safety, identify the functional substance.
Also confirm for functional substance by qualitative and quantitative analysis.
We have been entrusted with more than 800 non-clinical study since 2001.
We had a partnership with sites that are expert on non-clinical study to provide higher end services since 2012.
Also, we support to studies based on Good Laboratory Practice(GLP), for applying for TOKUHO, and for preparing for Marketing Notification of Foods with Functional Claims.
Laboratory Studies
- Safety Testing
- Single-Dose / Repeat-Dose Toxicity, Genotoxicity(Ames, Chromosomal Abnormality, Micronucleus, and more.,), Reproductive Toxicity, Teratogenicity, Carcinogenicity, Dermal Irritation, the Draize Testing, and more.,
- Animal Model Efficacy Testing
- Hypotensive Effect, Hypoglycemic Effect, Reducing Body Fat, Reducing Arthritis, Hypocholesterolemic effects, Antiallergic Effect(Atopic Dermatitis, Hay Fever, and more.,), Antitumor Effect, Reducing Hyperuricemia, and others
- Others
- Safety Testing of Cosmetics and Topical medication, In-Vitro-tests(Cell Culture, various types of enzymes), Genetic Analysis for the Efficacy, Customized Analysis, Insect Repellent Testing, and others
Intelligence & Technology Lab
Click to see what Intelligence & Technology Lab doesFood Ingredients Analysis ・ Functional Substance Research
We report results of analytical research for quality management of nutrient composition of food at low-cost and in short-term, cooperating with large inspection companies; LSI Medience Corporation and Techno Science Co., Ltd..
We perform following cooperating with Universities or sites which are expert in special analysis, developing Foods for TOKUHO, identifying the functional substance to develop Foods with Functional Claims, considering qualitative analysis and quantitative analysis.
Please feel free to contact us for any inquiries.
Inquiry of Food Ingredients Analysis
Please feel free to contact us for any inquiries !
E-mail to usResearch Support Service
We support your research, consulting on various types of studies for applying for TOKUHO, Foods for Specified Health Use, preparing for Notification of Foods with Functional Claims, Sales Promotion, and more.. In addition, we develop new criteria applied human clinical trial cooperating with Universities.
Consulting
Medical Experts and Statisticians will offer professional advice, guidance, and solutions to your research issue.
Cases
- Clinical Trials/Human Studies
- Functionality and Safety of food to develop food products
- The way of Evaluating Functionality and Safety of food
- Publishing Journal, Conference Presentation
- Foods for Specified Health Use, or Notification of Foods with Functional Claims
- Patent
- Offering professional advice to your technical issue
Support Preparation of Article
To apply for TOKUHO or submit Notification of Foods with Functional Claims, articles prove the efficacy based on the result of clinical studies and/or human clinical trials are required.
Also, we are welcome to support publishing in journal in English and/or Japanese.
Clinical Study Site Support Business
We make contracts with clinical study sites and give top priority to protection of the patient human rights and considering the safety. We support clinical study sites’ performance so that clinical trails are conducted appropriately and smoothly based on the ethical appropriateness and scientific rationality of research protocols approved by research ethics committee.
Main Service
- Support for the informed consent and patients’ schedule management.
- Support contact with patients
- Support management of research data
- Support to create the case report form
- Support clinical trials’ services at each sites
For detailed documents, any inquiries or comments about Foods with Health Claims development support business(Food CRO), please contact us via the inquiry form below.
Inquiry Form
